Millikan Oil Drop Experiment
Winter 2014
The Millikan Oil Drop experiment was the first experiment to determine the charge of an electron with any significance to the value. Other experiments had used water with ions in a vapor which produced mixed results. What sets the two different experiments apart is the liquids that were used to create the drops. Water tends to evaporate while in contact with air, changing the mass. The oil used in Millikan’s experiment was a non-volatile variety, causing the drops to retain their mass.
The apparatus that was used to conduct this learning modules was the Pasco Scientific Millikan Oil Drop Apparatus AP-8210A which is a scaled down version of the original one.
To perform the experiment, the non-volatile oil is sprayed into the chamber. Some of the oil drops find their way between two brass plates that are initially grounded. To find a drop that has a net charge on it, the plates become charged and drops that have a charge will rise or fall toward the oppositely charged plate. Finding a drop that has a free fall of approximately 1 millimeter per 20 seconds is ideal. To gather data, measure the distance the drop falls and the time it takes to do so. Then do the same while it rises. Taking these values, and including air pressure, temperature of the observational chamber, and the voltage across the plates, the charge can be found for a particular drop of oil.
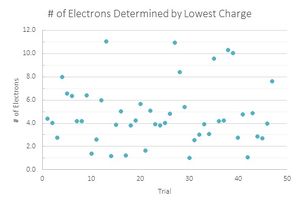
In my analysis of the data I recorded, I performed two parallel calculations. One was to divide all the charges that I had found from my data by the smallest one in the belief that this was the fundamental charge.
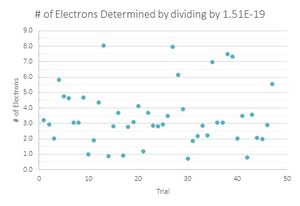
The second was to find a value that was close to the accepted value and divide all of the charges by that value.
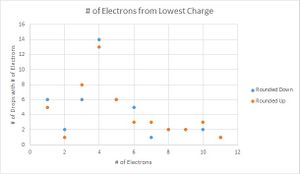
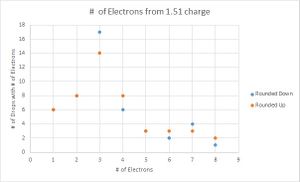
To determine which value for the charge of an electron was a better fit for my data, I then graphed the number of data points that were recorded for each number of electrons. Here I performed another parallel calculation where if the integer value of electrons ended in .4, .5, or .6, I would first round them down, then round them up for another data set.
Looking at the graphs, the charges that were divided by the lowest value acted sporadically. Where the number of drops that have one and three electron values are the same, the drops with two electrons are located in a trough, suggesting that this is not the value that is being sought. The second graph looks much more like what is expected, with the lower values increasing until three electrons and decreasing with very little variation in the data. Therefore the data suggest that 1.51*10^-19 C is the fundamental charge of an electron for my data.
Links