Electron Spin Resonance
Electron Spin Resonance (ESR) is a phenomenon, usefull for the investigation of materials which contain unpaired spins. Those are caused by various sources. One are not completely filled atomic shells, however, the more interesting ESR signals arise from different circumstances combined in the term defect. For instance, a important one is impurities of semiconductors or isolators which can trap electron or holes. Therefore this measuring technique is very powerfull evaluation of material properties and is used in lots of research fields in physics, chemistry and biology.
Physical background
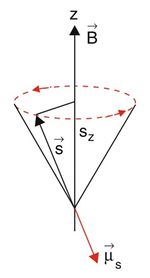
As the term Electron Spin Resonance already implies, the most essential part of the entire topic is the Spin, an intrinsic angular momentum of the electron. In an atom most of the electron are paired regarding spin. That is, each electron has a partner with an opposite spin, creating a zero net spin. Depending on the electron configuration of an atom or one step above in molecules and solids respectively, it is possible that there are a few or even many single electrons, being up to material.
Generally the magnetic moment of an electron is given by
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{\mu}=-\mu_B\left(\vec{L}+g_e\,\vec{S}\right) } ,
where Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_B } is the Bohr's magneton and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle g_e } the magnetogyric ratio, for a free electron it is Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle g_e=2.0023\approx 2 } . This formula includes the orbital angular momentum as well. With ESR it is only possible to detect and investigate the spin component of the total magnetic moment. Hence, in the further deviation we will look at the pure magnetic moment originating from the spin Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{S} } .
If the spin Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{S} } and its magnetic moment Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{\mu_S} } is placed in a externally applied magnetic field, the magnetic moment of the spin starts to precess around the axis of the magnetic field due to the torque
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{M}=\vec{\mu_S}\times\vec{B_Z} } .
As always in Quantum Physics the chosen axis of the magnetic field points in Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle z } direction.
In generell, the energy Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle E } of a magnetic moment Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{\mu} } is defined as
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle E=-\vec{\mu}\cdot\vec{B_Z} } .
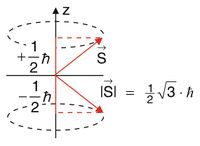
The magnetic moment Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{\mu} } is related to the spin Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \vec{S} } , which is a so called Quantum Vector with special properties:
- Absolute value: Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle |S|=\sqrt{s\left(s+1\right)} }
- Projection onto the z-axis: Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m_S=\pm\frac{\hbar}{2} }
Incedentally, those two quantities are the best way to distinguish the state of the electron, because only their operator commute and hence, are mesasurable at the same time. For instance, it is not possible to measure two of the three cartesian components of the spin vector simultaneously.
The equation for the energy of the electron's spin in an applied magnetic field contains a dot product between the magnetic moment and the B-field:
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle E=\mu_B\,g_e\,\vec{S}\cdot\vec{B_Z} }
It may seem to complicate the situation, but acutally it simplifies it a lot. We have chosen our magnetic field to point into the Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle z } direction. Since the spin is characterized by the projection onto the Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle z } axis, depicted by the Quantum number Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m_S } , the dot product cancels to
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle E=\mu_B\,g_e\,m_S\,B_Z }
with
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m_S=\pm\frac{\hbar}{2} } .
Bohr's magneton Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_B } and the magnetogyric ratio Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle g_e } are both natural constants at this point. If a external magnetic field Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle B_Z } is applied (positive value, because we picked the Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle z } orientation of our coordinate system in such a way), the electron's energy is reduced or raised, depending whether Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m_S } is negative or positive, respectively. The original single energy state, which we still get out of the equation for Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle B_Z=0 } , is split into two different energy states. Moreover, the energy difference
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Delta E=E_{+1/2}-E_{-1/2}=+\frac{1}{2}\,\mu_B\,g_e\,B_Z-(-\frac{1}{2}\,\mu_B\,g_e\,B_Z)=\mu_B\,g_e\,B_Z}
is proportional to the strength of the applied magnetic field. Therefore that difference can be tuned very precisely by the experimental setup, which is very important for the latter detection of the ESR signal.
For typical laboratory B-fields the energy difference Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Delta E } ranges in the microwave photon band, if you convert the energy to wavelength or rather frequency. This is the point, where the name of the entire phenomenon originates. Namely, if one irradiates the unpaired spin, which normally sits in the lower energy state (due to general energy minimization of nature), with the correct frequency, the energy gets absorbed, combined with a transition to the upper level. In this case, the energy of the photons match exactly the energy difference between both states. Physicist call this situation Electron Spin Resonance.
In formula language the fowolling becomes true;
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle h\,\nu=\mu_B\,g_e\,B_Z } ,
where Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle h } is Planck's constant and Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \nu } the frequency of the incident light.
Other than the magnetogyric ration Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle g_e } are all quantities either natural constants or measured values. Above I claimed that this value is a natural constant, too. Well, technically it is one, however, depends on the material and its compounds and especially spin stucture. Taking the previous equation as well as transferring it to an expression for the magnetogyric ratio
Failed to parse (MathML with SVG or PNG fallback (recommended for modern browsers and accessibility tools): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle g_e=\frac{h\,\nu}{\mu_B\,B_Z} }
yields material properties with regard to the spin configuration. For example, the comparison to the definite value of a free electron is a indicator for various substances' features.
.
.
.
TO BE CONTINUED
.
.
.

