Difference between revisions of "Slide Set-Up"
| Line 62: | Line 62: | ||
</td> | </td> | ||
<td> | <td> | ||
| − | <p>Retrieve around 10 micro-liters of microsphere solution. Make sure that this is very dilute. If you can see any opacity, it is too concentrated.</p> | + | <p>Retrieve around 10 micro-liters of diluted microsphere solution. Make sure that this is very dilute. For example, if you have a starting liquid microsphere solution with 10% solids, shake the original bottle to evenly disperse the beads then try a dilution of ~ 200:1 of deionized water (you may need to dilute even more). If you can see any opacity, it is too concentrated.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 107: | Line 107: | ||
<h4>Important notes:</h4> | <h4>Important notes:</h4> | ||
<ul> | <ul> | ||
| + | |||
<li> | <li> | ||
| − | + | Make sure to orient the slide so that the cover slip is facing towards the objective lens. In our case, the working distance of our objective lens is 0.2 mm. If your's is similar, the sample will be too far away to focus. | |
| − | |||
| − | |||
| − | Make sure to orient the slide so that the cover slip is facing towards the objective lens. In our case, the working distance of our objective lens is 0.2 mm. If your's is similar, the sample will be too far away to focus | ||
</li> | </li> | ||
<li> | <li> | ||
Revision as of 11:48, 24 November 2014
Back to main Optical Tweezers Page.
|
Place lens tissue paper above and below a microscope slide. |
|
|
To clean the slide, drop a small amount of methanol on top of the top piece of lens tissue. |
|
|
Slide the lens tissue across the microscope slide, making sure that the alcohol wipes across the entire slide. You may need something to hold the slide in place to keep it from... well, sliding. |
|
|
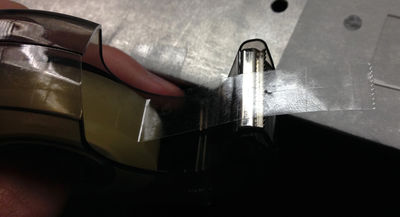
Cut a short piece of double sided sticky tape. Cut this piece in half length-wise so you get two, skinny pieces of tape. |
|
|
Put some vaseline on one side of both pieces of tape. |
|
|
Place both of these pieces of tape on the microscope slide about a centimeter away from each other with the vaseline sides facing each other. |
|
|
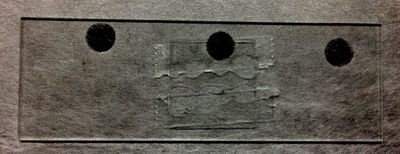
After cleaning a microscope cover slip in the same fashion as the slide, place a cover slip on top of the two pieces of tape. Be sure that the cover slip is secure. |
|
|
Retrieve around 10 micro-liters of diluted microsphere solution. Make sure that this is very dilute. For example, if you have a starting liquid microsphere solution with 10% solids, shake the original bottle to evenly disperse the beads then try a dilution of ~ 200:1 of deionized water (you may need to dilute even more). If you can see any opacity, it is too concentrated. |
|
|
Dry off any excess solution on the edges of the cover slip. |
|
|
Use top coat nail polish to seal the ends of the channels. |
|
|
Yay! |
Important notes:
- Make sure to orient the slide so that the cover slip is facing towards the objective lens. In our case, the working distance of our objective lens is 0.2 mm. If your's is similar, the sample will be too far away to focus.
- Take special care when applying nail polish near the microscope slide edges. If there is nail polish where the slide contacts the grooves of the slide mount, the nail polish may cause jamming or dry inside the grooves.
- To conserve microscope slides and to produce more stability, cleaving standard sized microscope slides into halves is a good idea.