Difference between revisions of "Slide Set-Up"
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
Back to main [[Optical Tweezers]] Page. | Back to main [[Optical Tweezers]] Page. | ||
| + | |||
| + | == Slide & Cover Slip Cleaning Procedure== | ||
| + | |||
<table border="1"> | <table border="1"> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:optical tweezer slide 1.jpg|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
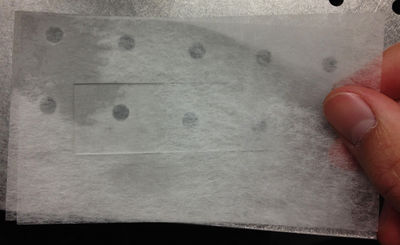
| − | <p>Place lens tissue paper above and below a microscope slide.</p> | + | <p>1. Place lens tissue paper above and below a microscope slide.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:optical tweezer slide 2.jpg|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p>To clean the slide, drop a small amount of methanol on top of the top piece of lens tissue.</p> | + | <p>2. To clean the slide, drop a small amount of methanol on top of the top piece of lens tissue.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:optical tweezer slide 3.jpg|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p>Slide the lens tissue across the microscope slide, making sure that the alcohol wipes across the entire slide. You may need something to hold the slide in place to keep it from... well, sliding.</p> | + | <p>3. Slide the lens tissue across the microscope slide, making sure that the alcohol wipes across the entire slide. You may need something to hold the slide in place to keep it from... well, sliding.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| − | + | </table> | |
| + | |||
| + | == Slide Preparation Procedure == | ||
| + | |||
| + | <table border="1"> | ||
| + | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 1.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
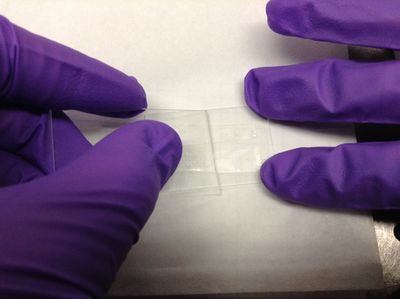
| − | <p>Cut a short piece of double sided sticky tape. Cut this piece in half length-wise so you get two, skinny pieces of tape.</p> | + | <p>1. '''Wear gloves''' to avoid getting skin oils on the sample. Cut a short piece of double sided sticky tape (Scotch brand for quality). Cut this piece in half length-wise so you get two, skinny pieces of tape. Stick the pieces of tape to a microscope slide half so that the pieces are parallel and separated by about 3-4 mm. </p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 2.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p> | + | <p>2. Scrape the tape down flat with another microscope slide. Be sure to scrape with a smooth, constant motion along the length of the tape. </p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 3.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p>Place | + | <p>3. Place a clean cover slip over the sticky tape to create a narrow channel that will become the flow cell. Using a rounded piece of plastic like the end of an ependorf sample tube, press the cover slip down firmly onto the tape to eliminate any air pockets (the tape will be more transparent as opposed to cloudy when firmly attached). Be sure not to press down too hard on the cover slip between the tape or you risk cracking the slip. If the slip cracks outside of the flow cell channel, the flow cell will still be usable, but be careful around sharp glass edges. </p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 4.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
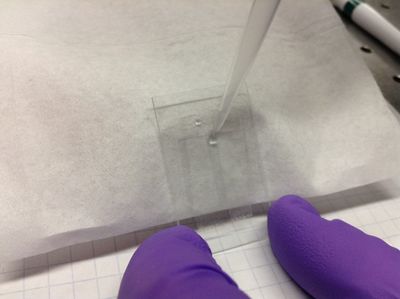
| − | <p> | + | <p>4. Incline the cell by tilting it against some small box. Using a micropipettor, flow ~10 microliters of deionized water into the flow cell to flush out any extraneous particles. If the liquid won't flow into the cell, stroke the cover slip over the bottom of the channel lightly downward to begin to "pull" the liquid into the cell.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 5.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p> | + | <p>5. Wipe off excess liquid with a Kimwipe using quick, light strokes to avoid pulling any liquid out of the flow cell. Any small bubbles that appear at the edges of the flow cell at this point will likely be permanent, although the cell will probably still be usable.</p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 6.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
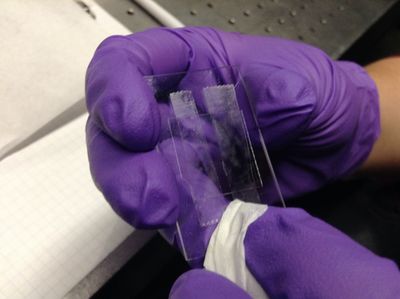
| + | <p>Flow ~10 microliters of diluted microsphere solution into the flow cell, again carefully and quickly wiping off the excess with a Kimwipe. </p> | ||
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 7.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p> | + | <p>7. Seal the ends of the flow cell channel with nail polish (sparkles serve to increase team morale).</p> |
</td> | </td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| − | <p> | + | <p>[[File:Slide prep 8.JPG|400px]]</p> |
</td> | </td> | ||
<td> | <td> | ||
| − | <p> | + | <p>8. Allow nail polish to dry. Sample should be ready to use. </p> |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</td> | </td> | ||
</tr> | </tr> | ||
| + | |||
</table> | </table> | ||
| Line 110: | Line 104: | ||
<li> | <li> | ||
| − | Make sure to orient the slide so that the cover slip is facing towards the objective lens. In our case, the working distance of our objective lens is 0.2 mm. If | + | The pieces of tape becoming cloudy is a sign that the flow cell liquid is leaking into the tape, and the sample will probably not be viable. |
| + | </li> | ||
| + | <li> | ||
| + | Make sure to orient the slide so that the cover slip is facing towards the objective lens. In our case, the working distance of our objective lens is 0.2 mm. If yours is similar, the sample will be too far away to focus. | ||
</li> | </li> | ||
<li> | <li> | ||
| Line 117: | Line 114: | ||
<li> | <li> | ||
To conserve microscope slides and to produce more stability, cleaving standard sized microscope slides into halves is a good idea. | To conserve microscope slides and to produce more stability, cleaving standard sized microscope slides into halves is a good idea. | ||
| + | </li> | ||
| + | <li> | ||
| + | Getting a working slide is possibly the most difficult part of the lab. Don't be discouraged! | ||
</li> | </li> | ||
</ul> | </ul> | ||
Latest revision as of 21:50, 4 September 2015
Back to main Optical Tweezers Page.
Slide & Cover Slip Cleaning Procedure
|
1. Place lens tissue paper above and below a microscope slide. |
|
|
2. To clean the slide, drop a small amount of methanol on top of the top piece of lens tissue. |
|
|
3. Slide the lens tissue across the microscope slide, making sure that the alcohol wipes across the entire slide. You may need something to hold the slide in place to keep it from... well, sliding. |
Slide Preparation Procedure
|
1. Wear gloves to avoid getting skin oils on the sample. Cut a short piece of double sided sticky tape (Scotch brand for quality). Cut this piece in half length-wise so you get two, skinny pieces of tape. Stick the pieces of tape to a microscope slide half so that the pieces are parallel and separated by about 3-4 mm. |
|
|
2. Scrape the tape down flat with another microscope slide. Be sure to scrape with a smooth, constant motion along the length of the tape. |
|
|
3. Place a clean cover slip over the sticky tape to create a narrow channel that will become the flow cell. Using a rounded piece of plastic like the end of an ependorf sample tube, press the cover slip down firmly onto the tape to eliminate any air pockets (the tape will be more transparent as opposed to cloudy when firmly attached). Be sure not to press down too hard on the cover slip between the tape or you risk cracking the slip. If the slip cracks outside of the flow cell channel, the flow cell will still be usable, but be careful around sharp glass edges. |
|
|
4. Incline the cell by tilting it against some small box. Using a micropipettor, flow ~10 microliters of deionized water into the flow cell to flush out any extraneous particles. If the liquid won't flow into the cell, stroke the cover slip over the bottom of the channel lightly downward to begin to "pull" the liquid into the cell. |
|
|
5. Wipe off excess liquid with a Kimwipe using quick, light strokes to avoid pulling any liquid out of the flow cell. Any small bubbles that appear at the edges of the flow cell at this point will likely be permanent, although the cell will probably still be usable. |
|
|
Flow ~10 microliters of diluted microsphere solution into the flow cell, again carefully and quickly wiping off the excess with a Kimwipe. |
|
|
7. Seal the ends of the flow cell channel with nail polish (sparkles serve to increase team morale). |
|
|
8. Allow nail polish to dry. Sample should be ready to use. |
Important notes:
- The pieces of tape becoming cloudy is a sign that the flow cell liquid is leaking into the tape, and the sample will probably not be viable.
- Make sure to orient the slide so that the cover slip is facing towards the objective lens. In our case, the working distance of our objective lens is 0.2 mm. If yours is similar, the sample will be too far away to focus.
- Take special care when applying nail polish near the microscope slide edges. If there is nail polish where the slide contacts the grooves of the slide mount, the nail polish may cause jamming or dry inside the grooves.
- To conserve microscope slides and to produce more stability, cleaving standard sized microscope slides into halves is a good idea.
- Getting a working slide is possibly the most difficult part of the lab. Don't be discouraged!