Difference between revisions of "Etching the PCB"
Aplstudent (talk | contribs) (→Testing the pH) |
Aplstudent (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | The APL has a tank of nasty copper chloride etchant (the polypropylene cereal box with nuclear green liquid inside). This functions via the following reactions. The obvious attraction of this process is that it produces no waste stream: The result of etching copper is more etchant! | + | The APL has a tank of nasty copper chloride etchant (the polypropylene cereal box with nuclear green liquid inside). This functions via the following reactions. The obvious attraction of this process is that it produces no waste stream: The result of etching copper is more etchant! Be very careful with the etchant as HCL is extremely acidic, also see the warnings below about the proper way to dispose of the etchant (do not pour it down the drain). |
Cu(s) + CuCl2 -> 2CuCl | Cu(s) + CuCl2 -> 2CuCl | ||
Revision as of 17:00, 21 February 2019
The APL has a tank of nasty copper chloride etchant (the polypropylene cereal box with nuclear green liquid inside). This functions via the following reactions. The obvious attraction of this process is that it produces no waste stream: The result of etching copper is more etchant! Be very careful with the etchant as HCL is extremely acidic, also see the warnings below about the proper way to dispose of the etchant (do not pour it down the drain).
Cu(s) + CuCl2 -> 2CuCl
CuCl + chlorinating oxidation -> CuCl2
Aside:
The tank contains a not insignificant amount of hydrochloric acid (the unmistakeable smell of which is detectable when it is open). However, the HCl is not free: It is almost entirely bound up in complexes with the copper chloride. Transition metal salts are known for being extremely colorful, and copper is no exception.
Dilute solutions of copper chloride in water are a serene blue (the copper ion coordinates with 6 waters). Intermediate concentrations are yellowish, and the addition of hydrochloric acid to a concentrated solution results in the copper ions forming a complex with two waters and four chloride ions (CuCl_4^{-2} \cdot 2H_2O), and it is this complex which gives our etchant its beautiful and almost hypnotically intense green hue.
The second reaction above is a summary of what is actually a rather complicated series of ion-complex reactions. The first reaction proceeds rapidly, while the second is the rate limiting factor (the insertion of a powerful oxidizer, like hydrogen peroxide, to the solution can speed this up).
This can be observed if the board is withdrawn entirely from the etchant solution to examine: Immediately, brownish liquid can be seen draining off of all exposed copper areas - these drops are etchant in which all available acid has been used up and copper (II) has saturated the solution with brown copper (I) ions.
This underscores why turbulent action is essential to the etching process: Like photographic development, etching is a surface reaction whose components come from a bulk fluid, and the bulk must be turbulently washed over the surface to continuously bring "new" etchant, unladen with copper I ions, within diffusion range of the surface.
Testing the CuCl2
Before using the CuCl2 you want to make sure that the etchant has the correct pH balance and the correct specific gravity. The ideal pH of the solution is < 1 and the ideal specific gravity is between 1.2393 and 1.3303.
Testing the pH
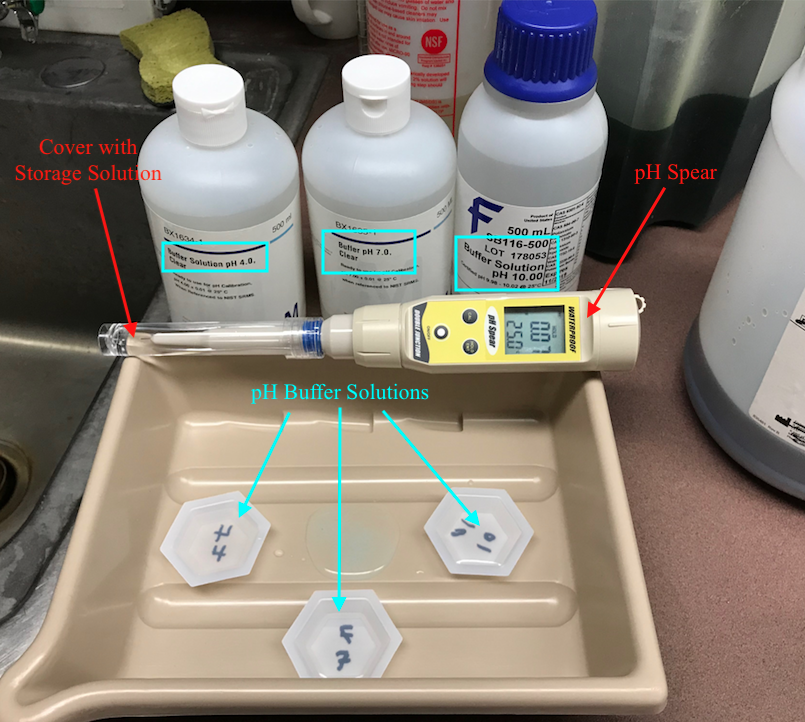
To test the pH you'll want to use a pH meter. In the APL we have one called a pH spear. This can be calibrated and then used to test the pH of the etchant. Calibration should be done once a week.
To calibrate the pH meter you will need 3 clear, liquid solutions called pH buffers. Each pH buffer has a precisely determined pH that can be used to calibrate the pH meter. First, put on eye protection and gloves. Then, set up the three buffer solutions in three labeled trays like the picture below (make sure to write on the bottom side of the trays to avoid changing the pH of the solution in the tray) and place the three small trays in a large tray to contain spillage or dripping. Also, make sure the solutions are about room temperature and then proceed to calibration.
When you are ready to calibrate the pH meter, turn on the meter by pressing the ON/OFF button. Next, carefully remove the storage cap and solution from the tip of the pH meter. Make sure the storage solution does not spill and place the cap in a secure location for later. Rinse the tip of the pH spear with deionized water to remove the storage solution from the tip. Be sure to rinse the pH spear with deionized water between every submergence in a different liquid to wash away the previous liquid. Next, press the CAL button to begin calibration. Submerge the tip of the pH spear 2cm - 3cm below the surface of the 4 pH buffer solution for 2 minutes (this allows the electrode in the tip to stabilize). The screen will display two numbers, the top number is the reading for the current pH and the bottom number is the pH the meter is being calibrated to. Ideally, for the pH 4 buffer solution the top number will gradually drop or rise to 4.01 while the bottom number remains at 4.01 (the bottom number will only ever display 4.01, 7.00, or 10.01 which correspond to the different buffer solutions).
After 2 minutes in the solution, without removing the tip from the solution, press the HOLD/ENT button, this will save the current calibration. Rinse the tip with deionized water and submerge the tip in the pH 7 buffer solution. Once again press enter after 2 minutes and rinse the tip. Finally repeat this process for the pH 10 solution and rinse the tip with deionized water.
Now that the pH meter is calibrated, dip the pH meter 2cm - 3cm into the etchant and record the pH. At the time of writing this the pH of the solution is 0.07, which is well below 1 and thus adequate. When you remove the pH Spear from the etchant the tip will have a small amount of bright green etchant on it, because the etchant is caustic and should not go down the drain (see warnings below) you do not want to immediately rinse the tip with deionized water. First, while wearing gloves, gently dry the green liquid from the tip with kimtech wipes. This will absorb the excess liquid and you can then rinse the tip with deionized water.
If you are finished with pH meter return the pH spear into the storage cap with the storage solution and appropriately dispose of your excess chemicals (do not just pour them down the drain).
Using the CuCl2
The action is slow at our room temp (22C) and may require as long as 30 minutes of agitation. Agitation *must* be continuous (and currently, by hand as we lack a bubbler). The etching process occurs at the surface and not in the volume, thus it is a diffusion limited process - agitation is *essential* or the metal simply forms a depletion zone adjacent to itself devoid of etching ions. Etching is complete once close inspections shows NO remaining exposed copper.
Note that the board cannot be left to etch unattended even with automatic agitation - the acid etches across as it cuts down (at a fairly small angle), but if left indefinitely it will eat *all* the copper off the board.
Once etching is complete, remove the board from the etchant. Try to shake as much etchant as possible back off into the tank before putting the board into a prepared small tray of sodium bicarbonate solution. This will immediately halt the etch action, and remove all remaining copper ions from the etchant solution that remained to the board. This is important because putting copper ions down the drain is very illegal:
https://nature.nps.gov/water/ecencyclopedia/assets/contaminant-pdfs/copper.pdf
Copper ion concentrations measured in single-digit parts per million are dangerous to aquatic life, and the required dilutions for copper ion contaminated water to go down the drain are concomitant: Our tank of etchant would contaminate several olympic swimming pools of water beyond legal release limits.
Under no circumstances allow any amount of green etchant solution to go down the drain! If any does, dump the sodium bicarbonate in immediately, chase it with a torrent of water, and pour additional bicarbonate in the sink straight from the box to 'kill it' before it enters the wastewater stream. If substantial spillage (more than a thimble full) occurs, get Dr. Boggs and call EHS immediately. A large release of copper ions down the drain threatens the entire city's wastewater treatment plant.
Gentle brushing will take any copper bicarbonate off the board. Once the board is clean, a remarkably small amount of poured acetone will strip the resist off of the copper exposing the beautiful tracks.